Iec 60601-1 / Iec 60601 1 2 4th Edition Announced Obelis
These start with EN 60601-1 Part One which covers basic safety and. This is a free 12 page sample.
Iec 60601 1 Medical Electrical Equipment Nl Tuv Rheinland
Please contact us with any questions.

Iec 60601-1. IEC 60601-1 is an International Standard and applies to the basic safety and essential performance of electrical medical equipment and electrical medical systems referenced as ME EQUIPMENT and ME SYSTEMS. The IEC 60601-1 and IEC 60601-1-2 Medical Electrical Equipment Package specifically address the electromagnetic compatibility of medcial electrical equipment. IEC 60601-1 is the primary standard governing medical device design Safety essential Performance so compliance with this standard has become requirement for bringing new medical devices into Market.
In conjunction with technical documentation and device. In 2005 the IEC released the 3rd edition which reflected a further change of perspective looking at means of protection MOP both for. The IEC 60601 was first published in 1977 then referred to as IEC 601 and handles the electrical safety of both mechanical and electrical issues.
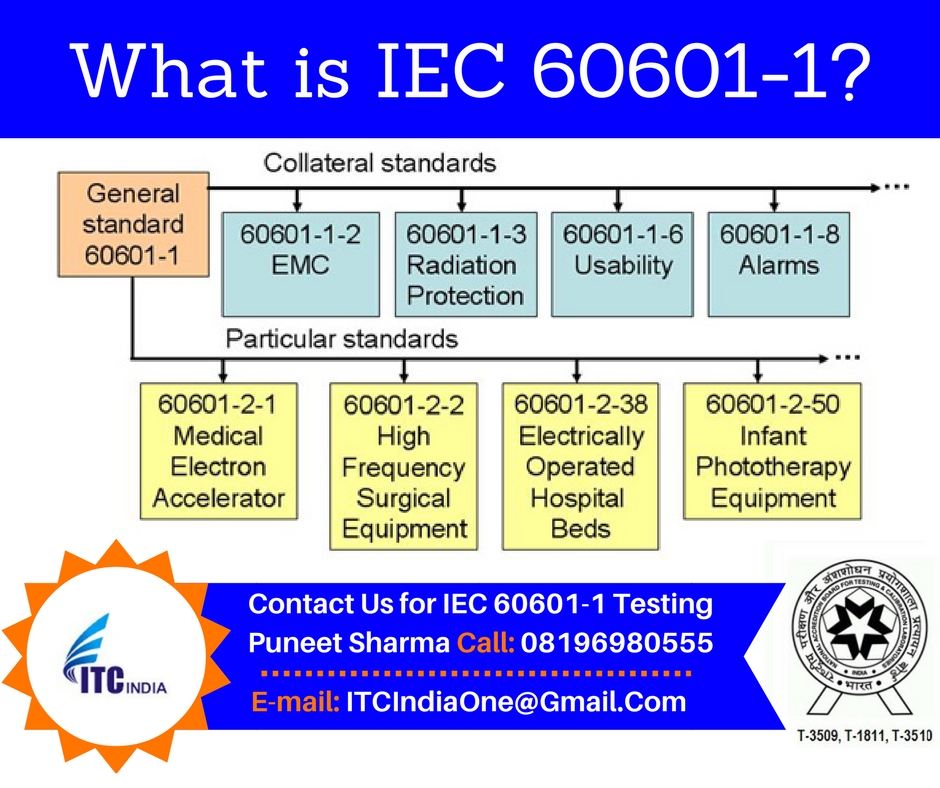
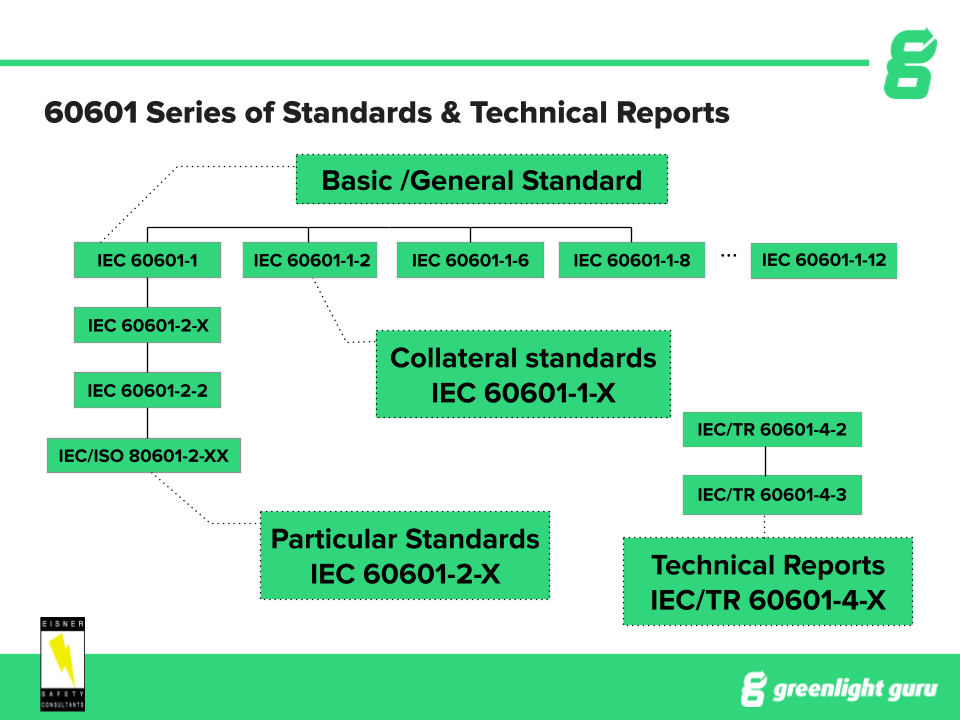
Instead it refers to the collateral standard IEC 60601-1-2. IEC 60601-1 32 Edition August 2020 - MEDICAL ELECTRICAL EQUIPMENT - PART 1. The changes included a revision and renumbering of clauses to align it with the 2005 edition of IEC 60601-1 and other ISOIEC editing requirements.
It applies regardless of whether the medical electrical equipment or medical electrical system is intended for use by a lay operator or by trained healthcare personnel. The force of this standard is to require two level of protection to guard the patient and operator from any injury. Finally the Annexes are a rich source of information in fact they comprise about 12 of the total size of the standard.
IEC 60601-1 Compliance Documents to evaluate medical electrical equipment to the applicable standards. IEC 60601-1 and IEC 60601-2 each built-up from a number of basic or collateral standards. The generated wave has an amplitude of up.
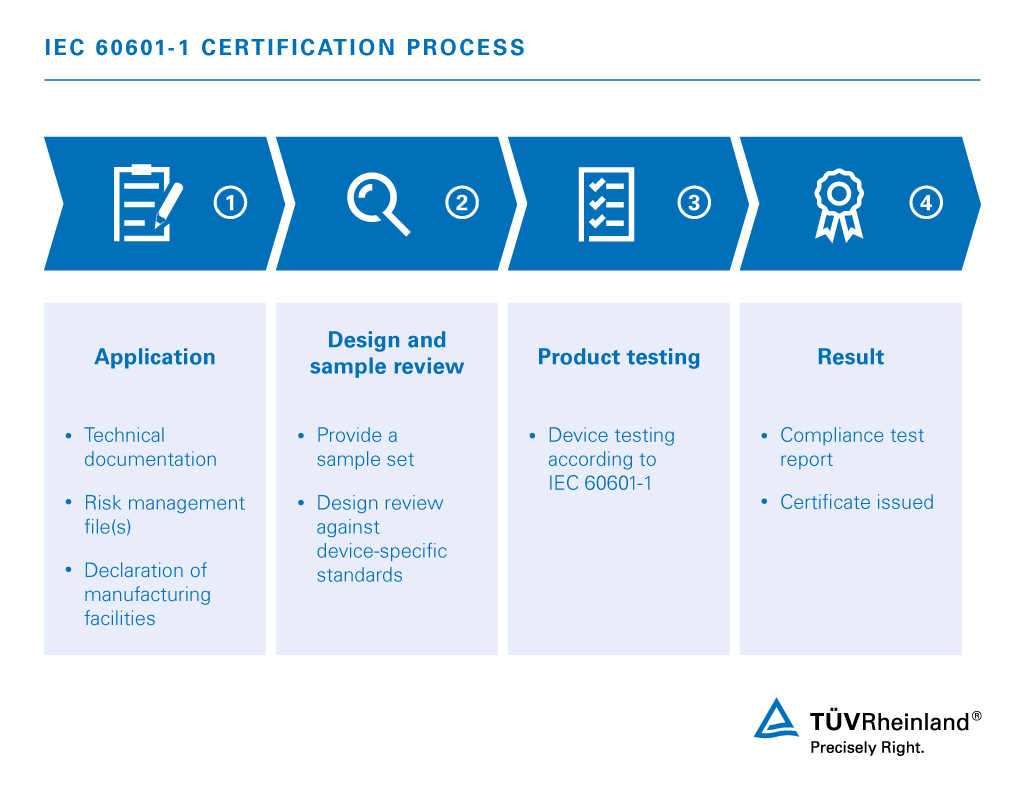
GENERAL REQUIREMENTS FOR BASIC SAFETY AND ESSENTIAL PERFORMANCE There is no abstract currently available for this document. Medical products must go through compliance testing and device approval before they can be marketed. The main change was in clause 4 where 3rd edition recognizes that IEC 60601-12005 implements a risk management process.
Our IEC 60601-1-2 testing clients range from established medical companies to startup entities producing a novel medical device and no single IEC 60601-1-2 testing product or project is the same. What is IEC 60601-1. This is especially true of smaller medical device companies.
EN 60601 is a group of standards which cover the safety essential performance and electromagnetic compatibility of medical electrical equipment and related systems. IEC 60601-1 is a series of technical standards for the safety and effectiveness of medical electrical equipment. Documentation of ISO 14971 compliant risk management practices must be clear throughout the product lifecycle and device labeling.
Overcrowding at testing labs is expected near dates of withdrawal. IEC 60601-1-112015 applies to the basic safety and essential performance of medical electrical equipment and medical electrical systems for use in the home healthcare environment. For certain types of medical electrical equipment these requirements are either supplemented or modified by the special requirements of a collateral or particular standard.
_____ EN 60601-12006 - 4 - Annex ZA normative Normative references to international publications. They fall into Informative and Normative ie. This standard can be used in part to show compliance under the US-FDA Canada-Health Canada and EU Medical Device Directive 200747EC regulations.
IEC 60601-1 mandates collateral particular and performance standards specific to the device type all of which are required for relevant certification schemes. IEC 60601-1 defines electromedical products as equipment provided with no more than one connection to a particular supply mains and intended to diagnose treat or monitor the patient under medical supervision and which makes physical or electrical contact with the patient andor detects such energy transfer to or from the patient. The 4 th edition is strictly one of these collateral standards known as IEC 60601-1-2.
The Medikzap is a modern electronic equipment generating a square wave current transferred to the body via two electrodes held in the hands. Collateral standard IEC 60601-1-x x representing a collateral. Access the full version online.
The 2nd edition published in 1988 focused on safety within the vicinity of a patient. The original IEC 60601-1 for medical devices was published in 1977. Standards play a paramount role in product design and development.
IEC 60601-1 edition 32 will cause manufacturers to abandon selling medical devices to countries that cannot compensate them well enough for the cost of retesting to edition 32. Of IEC 60601-1-2 are the same. Your labs ability and background knowledge in medical devices plays a huge role in the success and speed of your testing.
It is constructed from 2 parts. It is equivalent to the international standard IEC 60601 and comprises over 70 individual standards. Used together with IEC 60601-1 Test Report.
IEC 60601-12005A12012 contains requirements concerning basic safety and essential performance that are generally applicable to medical electrical equipment. The text of the International Standard IEC 60601-12005 was approved by CENELEC as a European Standard without any modification. The main IEC 60601-1 standard referred to in Europe as EN 60601-1 and in Canada as CSA 60601-1 is an umbrella for numerous subsidiary standards variously known as collateral or particular standards.
No specific requirements are listed in IEC 60601-1.

Iec 60601 1 6 Ed 3 2 B 2020 Third Edition Medical Electrical Equipment Part 1 6 General Requirements For Basic Safety And Essential Performance Collateral Standard Usability International Electrotechnical Commission 9782832287064 Amazon Com

Implementing The Iec 60601 1 Medical Electrical Equipment Standard Mddionline Com

Iec 60601 1 11 2015 Amd1 2020 Amendment 1 Medical Electrical Equipment Part 1 11 General

International Iec 60601 1 3rd Ed 2nd Amendment An Overview Ris World

Iec 60601 1 2 4th Edition Announced Obelis
Iec 60601 Medical Design Standards For Power Supplies Plianced Inc

Iec 60601 1 Medizinische Elektrische Gerate

Iec 60601 1 12 2014 Iec Standards Vde Publishing House

Iec 60601 1 8 2006 Amd2 2020 Amendment 2 Medical Electrical Equipment Part 1 8 General

Iec 60601 1 1 Ed 2 0 B 2000 Medical Electrical Equipment Part 1 1 General Requirements For Safety Collateral Standard Safety Requirements For Medical Electrical Systems Iec Tc Sc 62a Amazon Com Books

What Is The Iec 60601 Scope Medical Device Academy Medical Device Academy
Iec 60601 Medical Electrical Equipment Classification Faqs Medical Device Academy Medical Device Academy
15 Steps To Getting Approval For Iec 60601 1
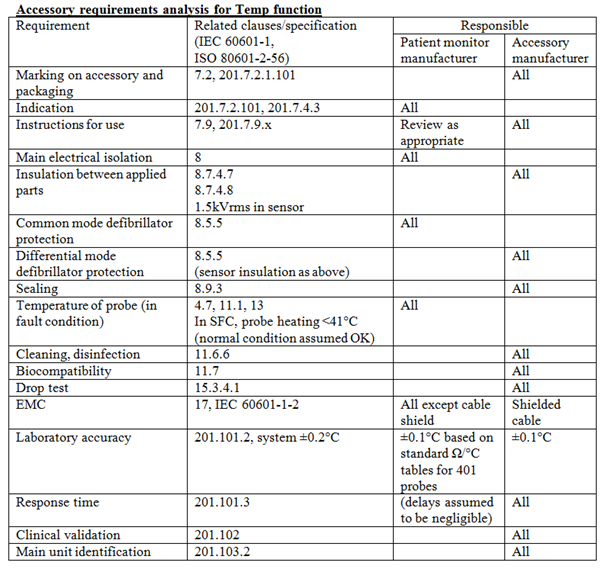
Iec 60601 1 And Accessories Medteq

Benefits Of Using Iec 60601 Compliant Components In Medical Devices